Acids and Bases Chemistry
Acids and bases are all over the place. Matter is something that has weight or mass and occupies space. Matter comes into three forms: solids, liquids, and gases. When mixed, acids can react with bases. When an acid and base are combined, the reaction makes two things happen. First the acid and base make a neutral liquid water. The product of the reaction between acids and bases is a solid substance, salt.
The most important acid of phosphorus is phosphoric acid, H3PO4 which is also called orthophosphoric acid. Pure phosphoric acid is a deliquescent crystalline substance with melting point 42oC. It is made by dissolving tetraphosphorus decoxide in water. Orthophosphoric acid forms three series of salts with one, two and three of its hydrogen atoms replaced by metal atoms. It is a weak acid, that is, it is a stable substance without effective oxidizing power.
How to Identify Acids and Bases?
An acid is a substance that increases the H+ ion concentration of the solution. A base is anything that increases the OH– ion concentration. Acids and bases that dissociate completely and stay dissociated are referred to as strong acid and bases. Dissociation is considered 100 percent and irreversible in the case of strong acids and bases, so a one-way reaction arrow is used in strong acid and baseline reactions.
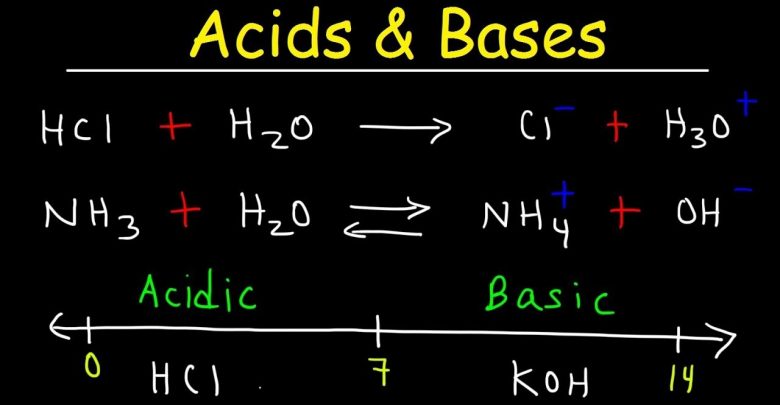
A strong acid is an acid that ionizes completely in water; it is a strong electrolyte. Hydrochloric acid HCl and nitric acid HNO3 are examples of strong acids. Using hydronium ion notation the respective equations are as follows.
HCl (aq) + H2O (l) → H3O+ (aq) + Cl– (aq)
HNO3 (aq) + H2O (l) → H3O+ (aq) + NO3– (aq)
A weak acid is an acid that only partly ionizes in water; it is a weak electrolyte. Examples of weak acids are hydrocyanic acid, HCN and hydrofluoric acid, HF.
HCN (aq) + H2O (l) → H3O+ (aq) + CN– (aq)
Uses of Orthophosphoric Acid
Phosphoric acid is also a compound of phosphorus with oxygen. Pure phosphoric acid is a colorless solid that resembles a crystal. Orthophosphoric acid, H3PO4, lies at the start of the main commercial route to all manufactured phosphorus compounds.
The biggest use of phosphoric acid is in the fertilizer industry. However, it has many other uses as well. Because phosphorus is quite poisonous, phosphoric acid is used to make pesticides and insecticides that protect crops and kill unwanted critters. In the metal industry, it is used to remove rust and to polish metals, such as aluminum. Phosphoric acid is also an ingredient in some cleaning products. Perhaps the use of phosphoric acid is most familiar for its addition to fizzy cola drinks.
